At Vasmed, we understand that successful medical device development goes beyond just technical expertise. Bringing a medical device to market requires meticulous planning, execution, and oversight. Vasmed provides comprehensive project management services to guide you through every stage of the development lifecycle, ensuring your project stays on track, within budget, and meets all objectives and regulatory requirements, while mitigating risks and optimizing efficiency.
Our Project Management Services
-
Project Planning & Scoping
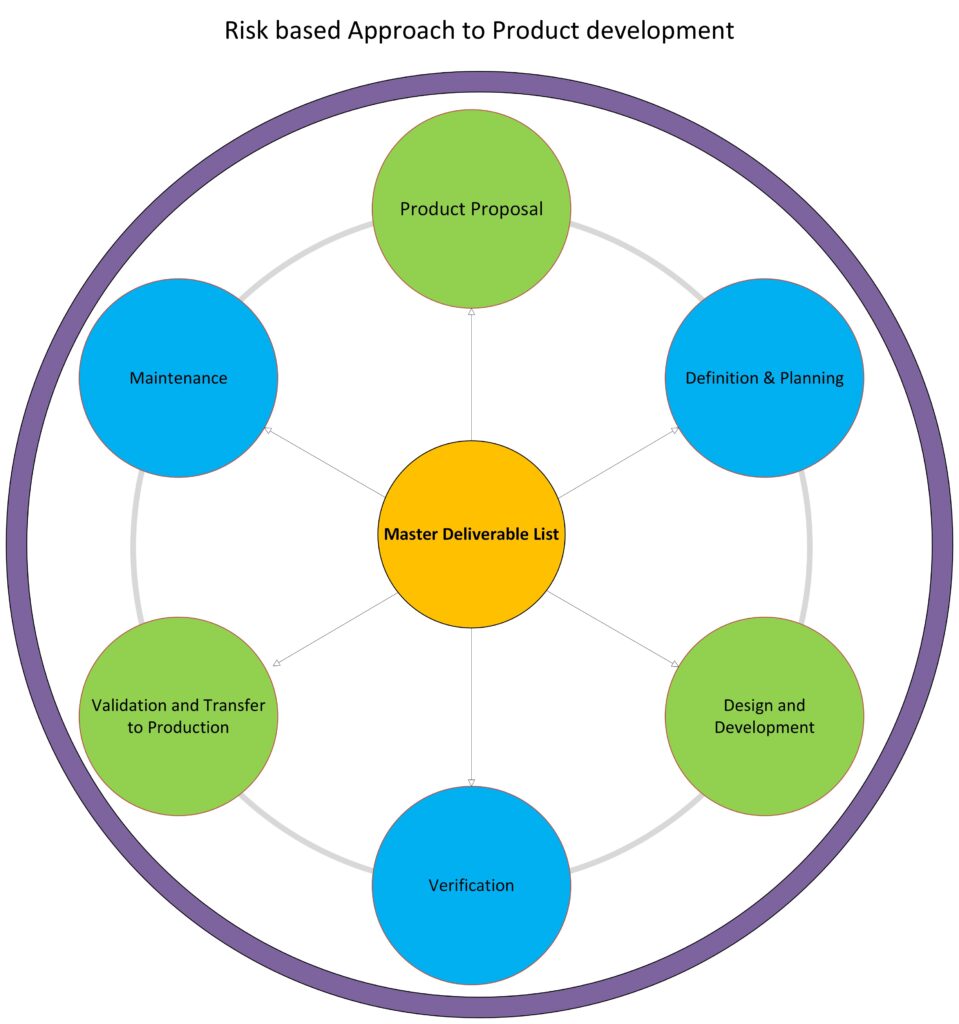
We work closely with you to define project objectives, scope, timelines, and budgets.. We carefully map out each phase of the project, from initial concept through to regulatory approval and market launch, allowing for precise tracking and management of progress.
-
Initial Planning: Define project scope, timeline, and deliverables.
-
Resource Allocation: Assign the right team members and tools to ensure that every aspect of the project is covered.
-
-
Cross-Functional Collaboration
Medical device development involves diverse teams with specialized expertise. Our project managers ensure smooth communication and collaboration across all disciplines, including engineering, regulatory, design, quality assurance, and manufacturing. We act as the central point of contact, coordinating between teams to maintain alignment, resolve challenges, and ensure project objectives are consistently met.
-
Team Coordination
Ensure that all departments and stakeholders are aligned on project goals.
-
Regular Communication
Facilitate ongoing discussions to keep all parties informed and to address any emerging issues promptly.
-
-
Risk Management
We identify and mitigate potential risks throughout the development process to minimize delays and cost overruns.
-
Team Management
We assemble and lead cross-functional teams, including engineers, designers, regulatory specialists, and clinical experts.
-
Budget & Schedule Tracking
We meticulously track project progress, monitor budgets, and proactively address any deviations from the plan.
-
Regulatory and Compliance Management
Medical devices must comply with stringent regulatory standards to ensure patient safety and market readiness. Our project management team has extensive experience navigating the complex landscape of FDA regulations, ISO standards, and international guidelines. We integrate regulatory requirements into the project timeline and workflow, ensuring compliance is maintained throughout development, testing, and production phases.
-
Regulatory Roadmap
Develop a timeline that includes necessary regulatory milestones and approvals.
-
Compliance Oversight
Ensure that all aspects of design, development, and testing meet the required standards, reducing the risk of costly delays due to compliance issues.
-
-
Quality Assurance & Control
We implement robust quality assurance and control measures to ensure product safety, efficacy, and compliance.
-
Communication & Reporting
We maintain clear and consistent communication with all stakeholders, providing regular project updates and reports.
-
Post-Launch Support and Monitoring
The project does not end at product launch. Our team continues to support you with post-market surveillance, collecting feedback, monitoring product performance, and ensuring any necessary updates or improvements are addressed. We ensure that any issues that arise after launch are managed swiftly, maintaining the device’s reliability and regulatory compliance.
-
Market Monitoring
Assist with post-launch data collection, analyzing user feedback, and tracking product performance.
-
Product Updates
Coordinate updates and improvements based on market feedback or regulatory changes.
-

Benefits of Our Project Management Approach
-
Reduced Development Time & Costs
Efficient project management minimizes delays and cost overruns, accelerating time-to-market.
-
Improved Quality & Compliance
Our rigorous processes ensure that your device meets the highest standards of quality and regulatory compliance.
-
Increased Success Rates
Our experienced project managers increase the likelihood of successful product launches.
-
Reduced Stress & Increased Focus
By managing the project, we free you to focus on your core competencies – innovation and product development.